Thank you for visiting the TRI Australia web page.
Here you will find information about our innovative medical research, clinical immersion, biotech and manufacturing, which together shape our bench-to-bedside ecosystem.
 The Translational Research Institute Australia (TRI) is Australia’s state‑of‑the‑art biomedical hub and a global leader in the effective translation of medical research and innovation into better healthcare outcomes.
The Translational Research Institute Australia (TRI) is Australia’s state‑of‑the‑art biomedical hub and a global leader in the effective translation of medical research and innovation into better healthcare outcomes.
Based in Brisbane, Queensland, TRI is home to more than 1100 researchers, clinicians, industry employees and corporate staff who support health discoveries from bench to bedside. There are also eight biotech companies, an international pharmaceutical company and nine service providers or peak bodies. These occupants thrive in TRI’s unique ecosystem, which provides incubator space for early-stage biotech companies to undertake discovery research, product development and prototyping.
In addition to the main building, TRI has a clinical trial facility within the Princess Alexandra Hospital, a paediatric clinical trial facility on the Queensland Children’s Hospital campus, and leases a large-scale bio-pharmaceutical manufacturing facility to Patheon by Thermo Fisher Scientific.
TRI is also building the future of translational manufacturing in the heart of Brisbane’s biosciences innovation precinct. Proudly funded by TRI and the Queensland Government, Translational Manufacturing@TRI (TM@TRI) will be Australia’s first scale-up biomedical manufacturing facility, supporting mid-stage biotech companies as they mature, expand and scale‑up product manufacturing.
TM@TRI is critical to allow biotech companies to remain based in Australia and to retain home-grown innovations and discoveries on shore.
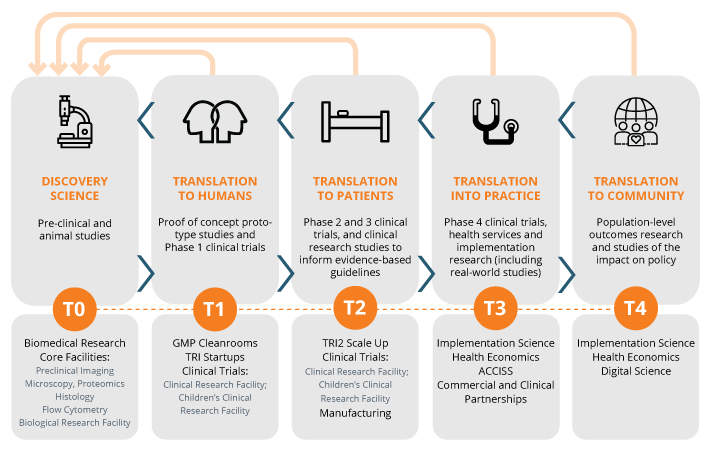
Translational Pathway: TRI’s mission is to improve the translation of innovative research into clinical practice. To achieve this, TRI provides world-class research facilities; links to clinicians and clinical trial facilities and expertise; links to industry, governments, and funding bodies; and an education and training program aimed at producing a skilled workforce for the translational pathway.
For more information about TRI’s translational research projects click here.
About TRI
The Translational Research Institute (TRI) is a world-class medical research facility, where leading Australian and international scientists and medtech start-up companies are focused on driving scientific excellence and innovation for healthier lives.
Unique in the Australian research landscape, TRI brings together researchers from its four shareholding partners: Queensland Health, The University of Queensland, the Queensland University of Technology, and Mater Research.
Click here to learn more about our partners.
The multi-partner institute was established to drive greater collaboration across Brisbane’s medical research sector, bringing researchers, clinicians and industry together to find answers to complex global health problems.
Adjacent to Brisbane’s Princess Alexandra Hospital, TRI is uniquely placed for discoveries, clinical trials, manufacturing and putting into practice new treatments, vaccines and diagnostics. This is all housed under one roof in a tertiary hospital setting.
Our Research: Research and collaboration are at the heart of everything TRI does. Our multi-partner institute was established to drive greater collaboration across Australia’s medical research sector. It is demonstrated by:
- the number of translational research projects underway
- programs focusing on translating innovation and involving collaboration across institutes, clinicians, technologies and disciplines, united by a common theme
- agreed areas of research themes
- access to state-of-the-art research equipment within the TRI Core Facilities (flow cytometry, microscopy, histology, proteomics, preclinical imaging, biological resources facility, gnotobiotics and GMP clinical manufacturing and training facilities known as T3 cleanrooms).
Click here to learn more about our translational research, innovation programs, areas of research and core facilities:
TM@TRI (Translational Manufacturing@TRI)

The TRI facility gives early-phase start up biotech companies time to establish, build, test and develop their products and provides a supportive environment to help advance medical discoveries to healthcare solutions. Once complete, Translational Manufacturing@TRI (TM@TRI) will be Australia’s first scale-up biomedical manufacturing facility supporting biotech companies as they mature and manufacture products for phase II and III studies.
TM@TRI will be the only facility in Australia where companies can perform their own product manufacturing in regulatory-compliant (TGA, FDA, EU) cleanrooms.
TM@TRI will leverage the research strengths and clinical synergies of TRI and its four shareholding partners.
TM@TRI will offer space for start-ups and SME in medtech and biotech to mature their business, validate and scale product manufacturing in cGMP facilities, prepare for clinical trials, and commercialise all under one roof.
Learn more about TM@TRI
TRI’s biomedical ecosystem
Industry@TRI: TRI is proud to house start-up biotech and medtech companies in the early stages of research and product development.
Our ecosystem is pivotal, aligns with TRI’s translational research pathway and includes researchers, clinicians, consumers, and biotech companies. Our model aims to bolster the biotech sector in its start-up, pre-revenue phase by providing a facilitated platform for companies while they are developing their medical interventions or technologies and conducting early phase clinical studies.
In addition, TRI also house companies which provide supportive services to the biotech sector such as VC funders, bioanalytic labs, and government funded accelerator/infrastructure bodies.
Learn more about our Industry@TRI







