TRI Flow Cytometry Facility
The TRI Flow Cytometry facility provides state-of-the-art instrumentation and expertise for cell sorting and analysis to advance translational to basic research. We offer both full-service and self-service facilities, with support and training, as well as cutting-edge research instruments and software resources.
Flow Cytometry is a powerful tool that assists researchers to analyse cells primarily through surface markers, but also intracellular, and secreted markers. The cell sorters allow researchers the opportunity to isolate populations of cells for cell culture, or further experimentation.
The Flow Cytometry Core through its user group, attempts to provide researchers with the opportunity to access a wide variety of analysers that can best optimise their experimental protocol.
THE TEAM
The team has extensive experience in biomedical research, project planning, flow cytometry panel design and experimental development, instrument operation and data analysis.
The facility is supported by two dedicated full time and two part-time staff with a combined experience greater than 50 years in flow cytometry/cell sorting.
What can we do?
- Monthly education seminar and instrument training
- Operator-assisted cell sorting
- Project conceptualisation and development
- Consultation and assistance with panel and experimental design
- Independent and assisted sample acquisition on cell analysers and data analysis
INSTRUMENTATION AND ANALYSIS SOFTWARE
The TRI Flow Cytometry facility houses 8 flow cytometry analysers (including an imaging flow cytometer), 3 droplet-based cell sorters, an AutoMACS Pro Separator and a haematology analyser/cell counter in rooms 4027/4027A. A suite of 6 workstations in room 4008 are available for the analysis of data using a wide range of flow cytometric software.
TRI is fortunate to have some of the most recently developed flow cytometry hardware housed within the TRI Flow Cytometry facility; the facility also has substantial capacity as to meet our researchers' needs. Ongoing upgrades will maintain all equipment and software at the highest possible level as to remain state-of-the-art.
|
For more information on instruments, services and facility charges contact the Senior Flow Cytometry Scientist: Dr Lucie Leveque-El Mouttie (07) 3443 7772 or [email protected] - |
 TRI Flow Cytometry facility room 4027/4027A
TRI Flow Cytometry facility room 4027/4027A
TRAINING
- Introduction to Flow Cytometry Course
Flow staff run a monthly 2-hour 'Introduction to Flow Cytometry' course open to new and existing users. This covers what a flow cytometer is, and how to choose dyes, set up panels, gating and compensation. New users must attend the 'Introduction to Flow Cytometry' course to be able to book a hands on instrument training.
Register for the Introduction to Flow Cytometry Courses >>
- Introduction to Spectral Flow Cytometry Course
If you are a new or existing user of the Cytek Aurora Spectral Flow Cytometer, the TRI Flow Cytometry facility strongly encourages you to register for the ‘Spectral Flow Cytometry course’ running every 3 months.
Register for the Introduction to Spectral Flow Cytometry course >>
- Instrument training
In order to get researchers onto instruments, hands on instrument training is supplied by request. Please note: Instruments cannot be booked until a certificate has been issued. The certificate represent your level of training/expertise on a particular instrument.
Book instrument training via PPMS
Stay tuned for talks on further topics from company application specialists.
EQUIPMENT AND SERVICES
ROOM 4027A - CELL ANALYSERS |
|
| BD FACSymphony A5 (SORP) | |
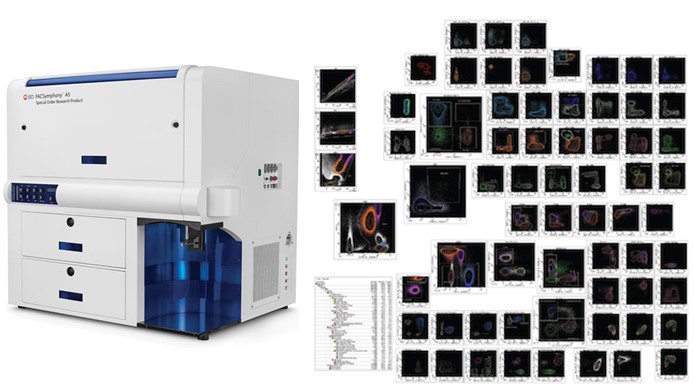 |
BD FACSymphony A5 (SORP): The BD FACSymphony A5 is similar to a BD LSR Fortessa X20 allowing greater choice of fluorochrome and panel flexibility. This instrument can be used for high number parameter panel (up to 28 colours) and also smaller panel especially where spread is an issue on the BD LSR Fortessa X20. This system is ideal to get breakthrough data in no time. 6 lasers (355nm, 405nm, 488nm, 561nm, 640nm & 785nm). 30 parameters (FSC, SSC and 28 fluorescence channels). Sample input tube types: 5 mL polystyrene tube and 96- or 384- well plates. BD FACSDiva |
| BD LSRFortessa X20 (SORP) (Three instruments) | |
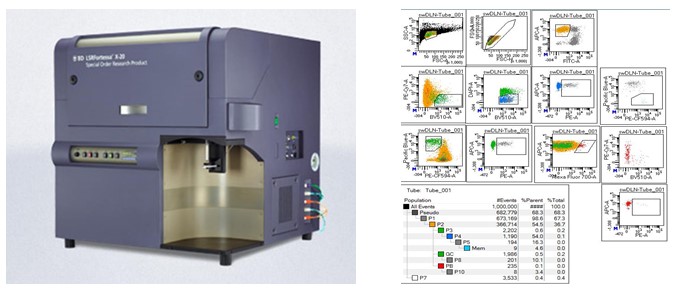 |
BD LSRFortessa X20 (SORP): The Fortessa X20 is ideal to set up panels of up to 18 fluorescent channels with 5 lasers including the UV laser to offer more options for fluorochrome choices and minimise spillover. 5 lasers (355nm, 405nm, 488nm, 561nm & 640nm) 20 parameters (FSC, SSC and 18 fluorescence channels) Sample input tube type: 5 mL Polystyrene tube and 96- or 384- well plates. BD FACSDiva All three BD LSRFortessa X-20 units are configured with the same laser and parameter filter configuration. BD LSRFortessa X20 (SORP) #1 BD LSRFortessa X20 (SORP) #2 (Everest) BD LSRFortessa X20 (SORP) #3 (Mauna Kea) |
| CYTEK AMNIS ImageStreamX MkII | |
 |
CYTEK AMNIS ImageStreamX MkII: The CYTEK AMNIS ImageStreamX MkII is an instrument capable of performing imaging cytometry, an application combining the advantages of flow cytometry and microscopy in one unit. 405nm, 488nm, 642nm and 785 nm laser 12- channel system (2 brighfield, SSC, up to 10 colours) 2-camera x20, x40 or x60 objectives EDF (Extended Depth of Field) module for enhanced spot count function INSPIRE acquisition software and IDEAS analysis software |
| Mindray BC-5000 Vet Auto Haemotology Analyser | |
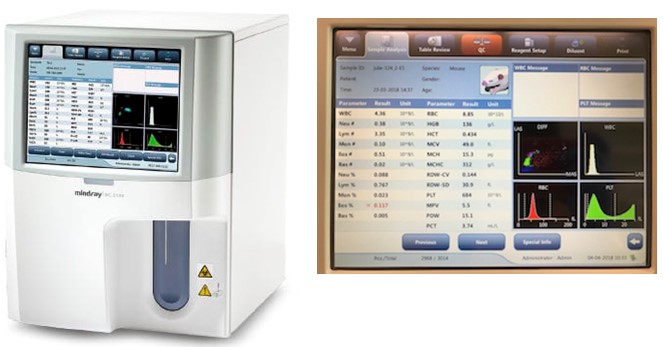 |
Mindray BC-5000 Vet Automated Haemotology Analyser: This instrument uses electrical impedance, chemical staining and low-, medium- and wide- angle laser scatter to perform complete blood counts (CBC's) on whole blood reporting 23 parameters. This instrument can analyse samples at a throughput of 60 samples per hour, and uses a minimal sample volume (15 uL) in whole blood mode. The Mindray enumerates absolute counts of platelets, erythrocytes and leukocytes (CBCs); being a 5-part diff unit it allows the differentiation of leukocytes into 5 subsets (Lymphocytes, Monocytes, Neutrophils, Eosinophils and Basophils). The instrument reports on numerous other haematological parameters (eg. haemoglobin levels, see below for full list). The instrument has 15 pre programmed settings for defined species of animals (e.g. Mouse, Rat, Rabbit, Ferret, Pig, Cow, Sheep, Goat, Monkey (human), Llama, Horse, Dog, Cat, Panda, Red Panda) and can accommodate an additional 20 custom-programmed species settings. It can be used for absolute cell counts on a variety of other samples other than blood. Parameters measured include: WBC, Lym%, Mon%, Neu%, Bas%, Eos%, Lym#, Mon#, Neu#, Eos#, Bas#, RBC, HBG, HCT, MCV, MCH, MCHC, RDW-CV, RDW-SD, PLT, MPV, PDW, PCT. |
| Beckman Coulter CytoFLEX-S (Two instruments) | |
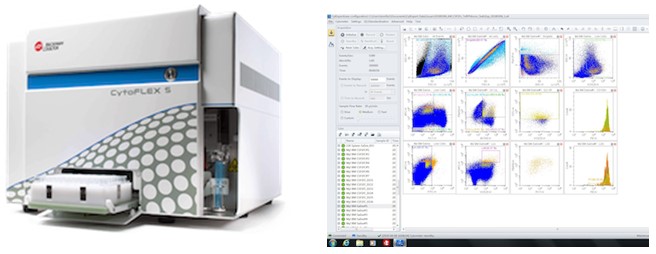 |
Beckman Coulter CytoFLEX-S: The CytoFLEX-S is a benchtop flow cytometer with extreme sensitivity and resolution. It has 13 fluorescent channels and 4 lasers. Easy to use, this machine is ideal for beginners and is the perfect machine to run cytokine beads assay with the plate loader. 4 lasers (405nm, 488nm, 561nm, 638nm) 15 parameters (FSC, SSC and 13 fluorescence parameters) Variety of sample tubes (1.5mL microfuge tubes, 5mL polystyrene and polypropylene tubes and has an adaptor for 1.2mL microtitre tubes) and 96 well plates Beckman Coulter CytoFLEX-S (CytoFLEX #1): This unit can be housed in a Class 2 Biohazard hood for analysis of Risk Category 2 pathogens. Beckman Coulter CytoFLEX-S (CytoFLEX #2 - GLYCOFLEX) Both Beckman Coulter CytoFLEX-S instruments are configured with the same laser and parameter filter configuration. |
| Spectral Flow Cytometer: Cytek Aurora | |
 |
Cytek Aurora: The Cytek Aurora is a spectral flow cytometer. Its new technology allows the detection of the full emission spectrum, which enables it to distinguish similar emission fluorochromes (FITC and GFP or APC and AF 647). Small to high number colour panel can be analysed on the instrument. A 40-color panel has been published by Cytek. 5 lasers (355nm, 405nm, 488nm, 561nm and 640nm). 67 parameters instrument (FSC, SSC-B, SSC-V and 64 fluorescence channels). Sample input tube type: 5 mL polystyrene tubes and 96-well plates to run samples. 40-tube rack or plate loader option. It requires no APD gain adjustment and no need to reconfigure optical filters for different fluorochromes. Fluorescence data is measured over a dynamic 6 decades of data. Autofluorescence extraction option. Live unmixing during acquisition. Small particle detection. SpectroFlo software for data acquisition |
ROOM 4027A - CELL SORTERS |
|
| Beckman Coulter MoFlo Astrios EQ | |
 |
Beckman Coulter MoFlo Astrios EQ: TRI Flow cytometry facility staff operate the Beckman Coulter Astrios EQ sorter within the flow core 7-laser (7-pinhole, 355nm, 405nm, 488nm, 532nm, 561nm, 592nm, 640nm) 26 parameters (FSC, SSC & 24 fluorescence channels). Jet-in-air based sorter 6-way cell sorting Different sort modes (independently operating on target sort populations) Sample input tube types: 5 mL polypropylene, 10 mL,15 mL and 50 mL tubes. 96- and 384- well plates. Temperature control The instrument is housed within a Biological Safety Cabinet allowing for sorting of human cells, human cell lines and virally-transduced cells. |
| BD FACSAria Fusion (SORP) (Two Instruments) | |
 |
BD FACSAria Fusion SORP: TRI Flow cytometry facility staff operate the two BD FACSAria Fusions sorter within the flow core. The 5-laser configuration is near identical to the BD LSRFortessa X-20 units configuration, allowing easy transfer of developed analysis panels to these instruments for sorting applications. 5-laser (5 pinhole, 355nm, 405nm, 488nm, 561nm and 640nm) 20 parameters (FSC,SSC and 18 fluorescence channels) Cuvette-based flow cell sorter allowing superior detection of some fluorophores compared to jet-in-air systems. 4-way cell sorting in a number of different sort modes Variety of collection tubes (5 mL polypropylene, 10 mL and 15 mL and 50 mL tubes) and 96- and 384- well plates. Temperature control allows for maintaining the environment of sample and sorted material. Both units are housed within Biological Safety Cabinets allowing for sorting of human cells, human cell lines and virally-transduced cells. BD FACSAria Fusion SORP (Fusion #1 5-Laser Leo) BD FACSAria Fusion SORP (Fusion #2 5-Laser Len) |
| Miltenyi autoMACS Pro Separator | |
 |
Miltenyi autoMACS Pro Separator: This instrument uses MACS (Magnetic activated cell sorting), a bead based technology available from Miltenyi to isolated cells directly from whole blood, bone marrow or virtually any cell type from any species subject to antibody availability. It can be used as an alternative or complement to droplet-based flow sorting. The unit is housed in a Bio-safety cabinet allowing sorting of human primary material. Several modes of purification are available that revolve around the principles of positive- or negative- selection. |
SOFTWARES |
|
|
FlowJo: FlowJo is a powerful statistical environment that is used for immunophenotyping, cell cycle, proliferation, kinetics studies, quantitative population comparison, high supervision analysis or plate screening assays to name a few. This software can be used for analysing a wide range of flow cytometric data collected on a variety of acquisition software platforms. With an intuitive interface, specialised analysis platforms and open-ended plugin architecture, FlowJo provides a rich analysis environment that is easy to use, versatile and extensible. The facility has 5 dongle licenses of this software available on PCs. BD FACSDiva: BD FACSDiva also allows analysis of flow cytometric data collected on acquisition/sorter units running FACSDiva in two ways. FACSDiva experiments can be exported as an 'Experiment' (.xml file format) and re-imported into FACSDiva for analysis, maintaining all previously generated plots, gates, hierachy information and statistical analyses. Alternative data in .fcs file format can be imported in the FACSDiva for analysis by normal means. FCS Express RUO: FCS Express is a fully integrated analysis, statistics, graphing and reporting tool for turning cytometry data into presentation-ready and publication-ready results. Flow cytometry and Imaging Flow cytometry data generated on any flow cytometer can be analysed. Unmixing spectral flow data and performing high supervision analysis are option available. It is a internet dongle licence available for one user at a time. Kaluza: Kaluza offers an intuitive analysis interface for the analysis of flow cytometric data collected on any flow cytometer. The facility has 5 copies of this software on analysis units licenced by a dongle-driven network-based site licence (if Kaluza analysis software doesn't open, please check that all workstations with Kaluza in the analysis suite area are turned on). SpectroFlo Software: Software for acquisition and analysis of data generated on the Cytek Aurora instrument. ModFit: Software for analysis of cell cycle distribution/DNA content. It is also capable of analysis of cell division where data was generated by dilution of dye-labelled cells (e.g. CFSE, CTV,etc.) IDEAS Software: Software for analysis of image-cytometry data generated on the AMNIS ImageStreamX MkII instrument. BD FCAP Array: BD software for the analysis of multiplex bead-based cytokine analysis kits. |
|







